FDA Approves Drug 10 Times Stronger than Fentanyl: What It Means for Drug Diversion Prevention
Just weeks after U.S. Food and Drug Administration (FDA) Chairman Dr. Raeford Brown warned that a new synthetic opiate would lead to “diversion, abuse, and death,” the FDA has approved the drug for use in managing acute pain in adults. And while FDA Commissioner Dr. Scott Gottlieb defended the decision and highlighted efforts being taken to “help prevent misuse and abuse,” the move raises new questions for care providers focused on drug diversion prevention. Sufentanil has been used since the 1980s, the pharmaceutical company AcelRx recently developed a sublingual tablet version of the drug called Dsuvia, which is 10 times stronger than fentanyl. On Oct. 12, the FDA advisory committee voted 10-3 in favor of approving the drug.
So how might this affect care providers concerned about new opioids being approved by the FDA in the midst of a massive opioid epidemic?
The FDA approval of Dsuvia comes at critical moment in healthcare, when opioid addiction rates and deaths are at all-time highs. In 2017, the U.S. Department of Health and Human Services (HHS) declared the opioid crisis a public health emergency. And according to the HHS’ Substance Abuse and Mental Health Services Administration, about 11.5 million Americans age 12 and older misused prescription pain medicine in 2017. In addition:
- 116 people die every day from opioid-related drug overdoses
- 42,249 people died from overdosing on opioids in 2016
- 21 million people have an opioid misuse disorder
These sobering statistics all contribute to the fact that 40 percent of opioid overdose deaths involve prescription opioids – an epidemic that is attributed to $504 billion in economic costs.
The Opioid Epidemic Driving Drug Diversion in Healthcare
Opioid theft in the care setting is not a new concept, but stories of drug diversion in the care setting have increased over the past decade, with major implications to patient safety:
- Ex-hospital worker gets 39 years for causing hepatitis C outbreak
- Registered nurse charged with stealing drugs intended for patients
- Colorado care workers jailed for stealing drugs had hopped from hospital to hospital
Drugs like Dsuvia can pose a diversion risk in a care setting and should be handled with patient safety in mind. Up to 15 percent of healthcare workers are addicted to drugs or alcohol, compared with 8 percent of the general population. And because of their proximity to narcotics and the availability of those drugs in a care setting, care workers hooked on opioids will often divert prescription drugs to avoid withdrawal. These prescription drugs can be diverted at any point along the supply chain or clinical workflow from manufacturers to distributors, pharmacies, hospitals, or other healthcare organizations.
The FDA has taken steps to reduce the likelihood of Dsuvia abuse, including a single-use dose applicator and a “black box” warning. It’s also only available for home use or through retail pharmacies. However, Dsuvia’s potency may motivate care workers to divert the drug – a single pill can be abused over a long period of time, and, as a result, may have a high value on the black market.
How Care Providers are Working to Help Stop Drug Diversion and Ensure Patient Safety
Due to its effects on patients, care providers, and even drug diverters themselves, the U.S. Drug Enforcement Administration (DEA) has cracked down on drug diversion; the agency has made over 54 enforcement actions and 283 administrative actions in the first half of 2018 alone. It recently made statements that opioid diversion prevention starts with the care provider. Plus, fines from the DOJ and DEA are becoming bigger – with a $4.3 million settlement recorded in 2018 – and the impact on patient care is significant. In response, top organizations are creating or growing drug diversion monitoring programs to combat diversion in their facilities.
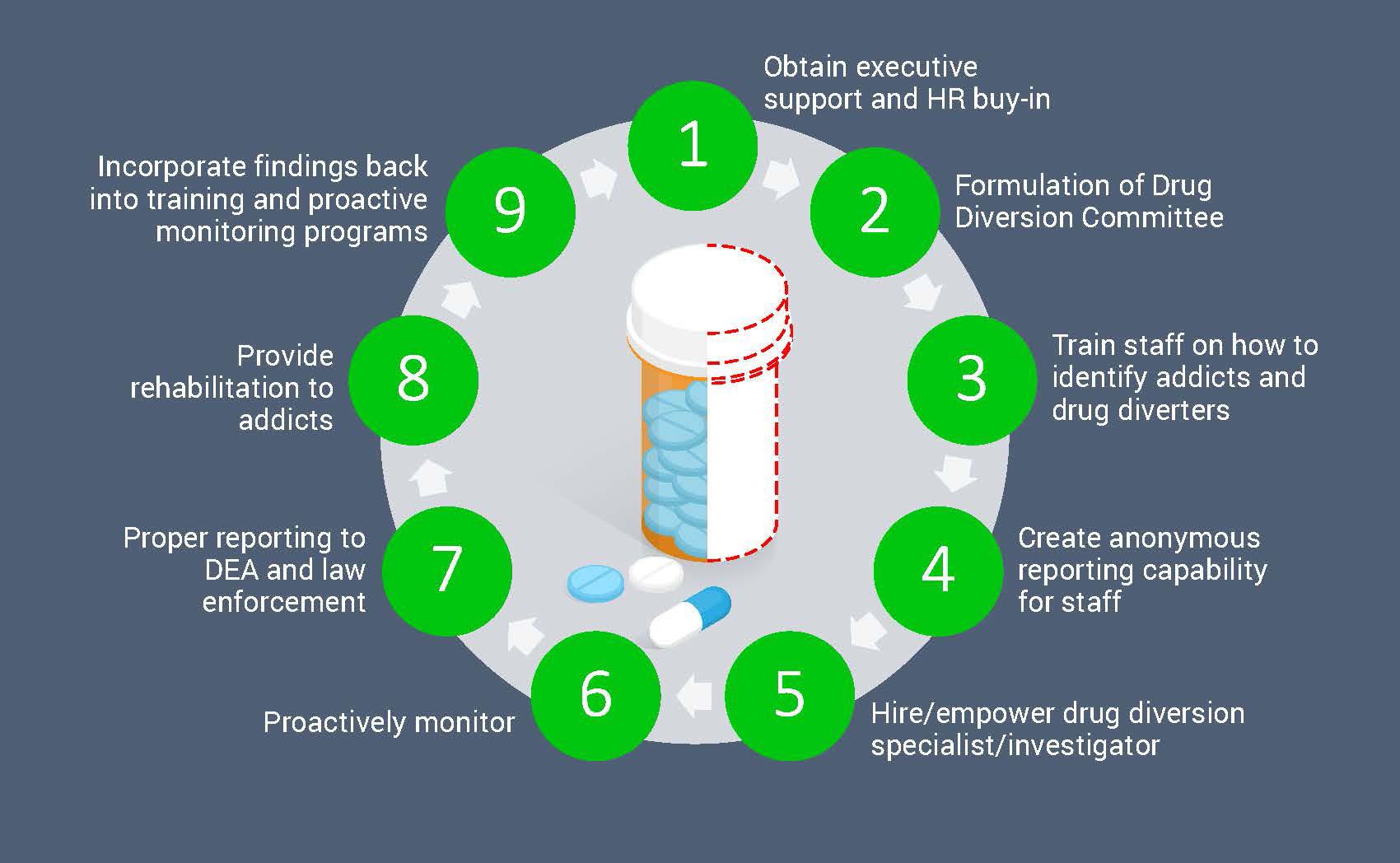
To create a full lifecycle drug diversion monitoring program, care providers need a strategic and proactive strategy. Tools, technology, and a culture-first focus can help stop incidents from occurring in the first place. Healthcare organizations should manage the full lifecycle of security incidents by detecting costly and damaging behavior like drug diversion, investigating the behavior, documenting the investigation, and more from one single platform that integrates with your EHRs and other mission-critical applications. By doing so, you can create a culture of security and patient safety while driving diversion out of your facility.